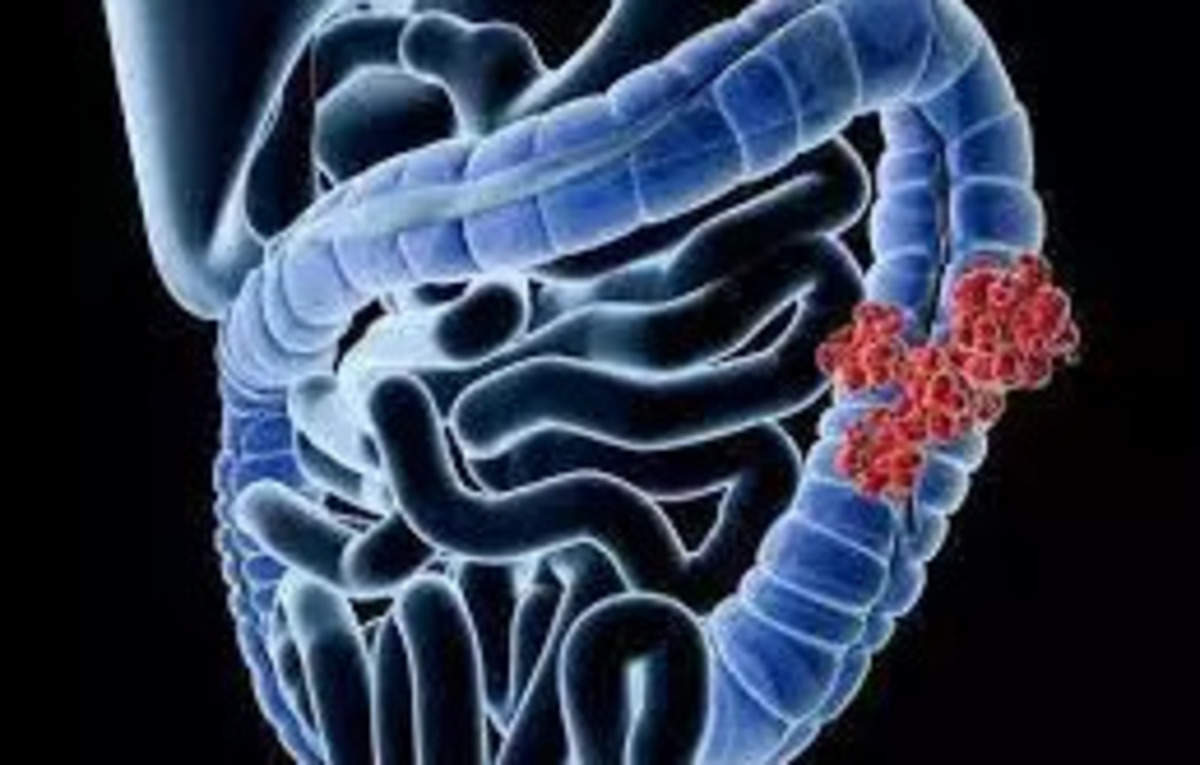
Washington: According to a new study, A new artificial intelligence model may bring much-needed clarity to physicians prognosticating and deciding treatment for patients with colorectal cancerthe second deadliest cancer in the world.
The study was published in the journal ‘Nature Communications’.
The new tool accurately predicts how aggressive a colorectal tumor is, how likely the patient is to survive with and without disease recurrence, and what the optimal therapy for them might be.
Having a tool that answers these questions could help clinicians and patients navigate this cunning disease, which often behaves differently even among people with similar disease profiles receiving the same treatment, and could ultimately save some of the 1 million lives that colorectal cancer claims each year.
The researchers say the tool is meant to enhance, not replace, the human experience.
“Our model performs tasks that human pathologists cannot do based on image display alone,” said study co-senior author Kun-Hsing Yu, an assistant professor of biomedical informatics at the HMS Blavatnik Institute. Yu led an international team of pathologists, oncologists, biomedical informaticians, and computer scientists.
“What we anticipate is not a replacement of human pathology expertise, but rather an augmentation of what human pathologists can do,” Yu added. “We hope this approach will augment the current clinical practice of cancer treatment.”
The researchers caution that the prognosis for any individual patient depends on multiple factors and that no model can perfectly predict the survival of any given patient. However, they add, the new model could be useful in guiding clinicians to follow up more closely, consider more aggressive treatments, or recommend clinical trials testing experimental therapies if their patients have poorer prognoses as assessed by the tool.
The tool could be particularly useful in resource-limited areas both in this country and around the world where advanced pathology and tumor genetic sequencing it might not be readily available, the researchers noted.
The new tool goes beyond many current AI tools, which primarily perform tasks that replicate or optimize the human experience. The new tool, by comparison, detects and interprets visual patterns in microscopic images that are imperceptible to the human eye.
The tool, called MOMA (for Multi-omics Multi-cohort Assessment) is freely available to researchers and clinicians.
The model was trained on information collected from nearly 2,000 colorectal cancer patients from various national patient cohorts that together include more than 450,000 participants: the Health Professionals Follow-up Study, the Nurses’ Health Study, the Cancer Genome Atlas Programand the NIH PLCO (prostate, lung, colorectal, and ovarian) cancer screening trial.
During the training phase, the researchers fed the model information about the age, gender, cancer stage, and patient outcomes. They also gave him information about the genomic, epigenetic, protein, and metabolic profiles of the tumors.
The researchers then showed the model pathology images of tumor samples and asked it to look for visual markers related to tumor types, genetic mutationsepigenetic alterations, disease progression and patient survival.
The researchers then tested how the model might work in “the real world” by feeding it a previously unseen set of images of tumor samples from different patients. They compared their performance with actual patient outcomes and other available clinical information.
The model accurately predicted patients’ overall survival after diagnosis, as well as how many of those years they would be cancer-free.
The tool also accurately predicted how an individual patient might respond to different therapies, based on whether the patient’s tumor harbored genetic mutations that made the cancer more or less likely to progress or spread.
In both areas, the tool outperformed human pathologists, as well as current AI models. The researchers said the model will be updated regularly as the science evolves and new data emerges.
“It is critical that with any AI model, we continually monitor its behavior and performance because we may see changes in disease burden distribution or new environmental toxins that contribute to cancer development,” Yu said. “It’s important to augment the model with new and more data as it emerges so that its performance never lags behind.”
Discern telltale patterns
The new model takes advantage of recent advances in tumor imaging techniques that offer unprecedented levels of detail, yet remain imperceptible to human evaluators. Based on these details, the model successfully identified indicators of how aggressive a tumor was and how likely it was to behave in response to a particular treatment.
Based on just one image, the model also identified features associated with the presence or absence of specific gene mutations, something that typically requires genomic sequencing of the tumor. Sequencing can be time consuming and expensive, especially for hospitals where such services are not routinely available.
In precisely such situations, the model could provide timely decision support for treatment choice in resource-limited settings or in situations where tumor tissue is not available for genetic sequencing, the researchers said.
The researchers said that before the model is deployed for use in clinics and hospitals, it needs to be tested in a prospective randomized trial evaluating the tool’s performance in real patients over time after initial diagnosis. Such a study would provide the gold standard demonstration of the model’s capabilities, Yu said, by directly comparing the tool’s performance in real life using only images with that of human doctors using knowledge and test results to that the model does not have access to.
Another strength of the model, the researchers said, is its transparent reasoning. If a doctor using the model asks why he made a certain prediction, the tool could explain her reasoning and the variables she used.
This feature is important to increase the confidence of doctors in the AI models they use, Yu said.
Measurement of disease progression, optimal treatment
The model accurately identified imaging features related to differences in survival.
For example, he identified three image features that portended worse outcomes:
– Higher cell density within a tumor.
– The presence of supporting connective tissue around the tumor cells, known as the stroma.
– Interactions of tumor cells with smooth muscle cells.
The model also identified patterns within the tumor stroma that indicated which patients were more likely to live longer without cancer recurrence.
The tool also accurately predicted which patients would benefit from a class of cancer treatments known as immune checkpoint inhibitors. While these therapies work for many colon cancer patients, some experience measurable benefit and have serious side effects. Therefore, the model could help doctors personalize treatment and avoid patients who would not benefit, Yu said.
The model also successfully detected epigenetic changes associated with colorectal cancer. These changes, which occur when molecules known as methyl groups bind to DNA and alter its behavior, are known to silence tumor-suppressing genes, causing cancers to grow rapidly. The model’s ability to identify these changes marks another way that it can inform choice of treatment and prognosis.