Prolonged suckling reduces high-fat diet-induced weight gain
To determine whether extended suckling in rats, modelling prolonged breastfeeding in human infants, could exert a long-term influence on the management of energy intake, litters born to chow-fed dams were weaned either at 3 weeks after birth (postnatal day 21, standard weaning or SW) or at 4 weeks (delayed weaning or DW). SW and DW pups were subsequently maintained on a CD or HFD until they were 18 weeks old (Fig. 1a).
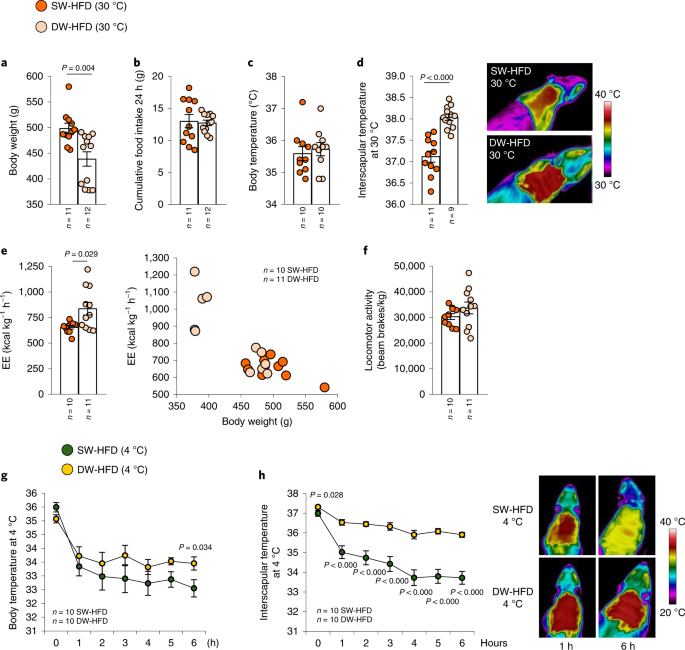
a, Timeline of the experimental protocol. b–h, Effects of delayed weaning on body weight (b); fat mass (c); non-fat mass (d); energy expenditure (EE) in light and dark phases and as a function of body weight (e); energy intake over 24 h (f); glucose tolerance (g); and insulin response (h). i–k, In rats fed an HFD, effect of delayed weaning on response to ICV administration of leptin (3 μg per rat) in terms of body weight change (i), cumulative food intake (j) and MBH protein levels of pSTAT3, STAT3, pPI3K, PI3K, pAKT, AKT, pERK and ERK (k). Protein data were expressed as percentages in relation to control (SW-HFD vehicle) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences between groups are indicated by the following colours: black, SW-HFD versus DW-HFD; green, SW-CD versus SW-HFD; violet, SW-CD versus DW-CD; red, DW-CD versus DW-HFD. Statistical differences were determined by one-way analysis of variance (ANOVA; normal data and homogeneity of variances) followed by Tukey’s post hoc multiple-comparison test (b–d, f and j) or a two-sided Student’s t-test (normal data; g and h), a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; e, i and k), or an analysis of covariance (ANCOVA) with body weight as a covariate (e). AUC, area under the curve.
Source data
Rats subjected to DW and given a chow diet (DW-CD) did not differ in terms of body weight from those subjected to SW and the same diet (SW-CD; Fig. 1b and Extended Data Fig. 1a). However, when DW rats were fed an HFD (DW-HFD), their body weight was substantially lower than that of SW animals that were kept on an HFD (SW-HFD) for the same period of time (Fig. 1b and Extended Data Fig. 1a).
In keeping with these results, an analysis of body composition using quantitative nuclear magnetic resonance (NMR) revealed that SW-CD and DW-CD rats had similar fat and non-fat mass values (Fig. 1c,d), but DW-HFD rats accumulated notably less fat mass than SW-HFD animals (Fig. 1c), while non-fat mass was unchanged between the two HFD groups (Fig. 1d).
Similar results were obtained when visceral and gonadal adipose tissues (VAT and GAT) were weighed (Extended Data Fig. 1b). In accordance with the lower fat content, energy expenditure was also higher in DW-HFD than in SW-HFD rats in both light and dark phases (Fig. 1e). These changes in energy expenditure between SW-HFD and DW-HFD rats were not associated with differences in locomotor activity, respiratory quotient (Extended Data Fig. 1c,d) or food intake (Fig. 1f). DW-HFD rats also showed reduced circulating levels of triglycerides, cholesterol, non-esterified fatty acids and leptin (Extended Data Fig. 1e–h), as well as improved glucose tolerance and enhanced insulin sensitivity when compared to SW-HFD animals (Fig. 1g,h and Extended Data Fig. 1i).
Next, we evaluated the phenotype of SW-HFD and DW-HFD rats in a thermoneutral environment (30 °C) to eliminate the extra metabolism necessary to maintain body temperature at lower ambient temperatures. Consistent with the results above, body weight was reduced while food intake was unchanged in DW-HFD rats (Fig. 2a,b). However, while body temperature was similar between the two groups (Fig. 2c), DW-HFD rats displayed a higher interscapular temperature than SW-HFD rats (Fig. 2d). Under thermoneutral conditions, energy expenditure was higher in DW-HFD rats, without any difference in locomotor activity (Fig. 2e,f). We then conducted a cold-exposure test to assess the thermogenic response of BAT and found that DW-HFD rats preserved their whole-body and interscapular temperatures better than SW-HFD rats at 4 °C (Fig. 2g,h).
a–f, Delayed-weaning effects in rats fed an HFD under thermoneutral conditions on body weight (a); cumulative food intake (b); body temperature (c); infrared thermal images and quantification of BAT interscapular temperature (d); energy expenditure (e) and locomotor activity (f). g,h, Delayed-weaning effects in rats fed an HFD and exposed to cold (4 °C) for 6 h on body temperature (g) and infrared thermal images and quantification of BAT interscapular temperature at 4 °C (h). Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided Student’s t-test (normal data; c–h), a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; a and b), or an ANCOVA with body weight as a covariate (e).
Source data
It should be noted that, an extra week of HFD feeding is present in the SW-HFD group. To investigate whether the extra week of HFD could have implications in the observed phenotype, the body weight data were also reanalysed by comparing the body weights of SW-HF and DW-HFD groups accordingly to the weeks of exposure to an HFD instead of weeks of age. It was found that DW-HFD rats still presented lower body weight gain, and lower fat mass than SW-HFD rats (Extended Data Fig. 2). Altogether, these findings indicate that prolonged suckling increases the thermogenic activity of BAT in HFD offspring, increasing energy expenditure and ultimately reducing weight gain in a feeding-independent fashion.
Prolonged suckling increases leptin sensitivity in high-fat diet-fed rats
To evaluate whether prolonged breastfeeding could improve leptin sensitivity in obesity, both SW-HFD and DW-HFD rats were given an intracerebroventricular (ICV) injection of either vehicle or leptin at a dose of 3 µg, known to induce a decrease in feeding and body weight in CD-fed rodents20.
Leptin ICV injection did not induce any change in body weight (Fig. 1i) or food intake (Fig. 1j) in SW-HFD animals, in keeping with the leptin resistance observed in animals and humans with diet-induced obesity (DIO)21,22. In contrast, when administered to DW-HFD animals, leptin significantly decreased body weight (Fig. 1i) and food intake over 24 h (Fig. 1j). After collecting the hypothalamus of the different groups of rats, we assessed the impact of DW on the hypothalamic leptin receptor signalling pathway, expected to be impaired in HFD rats. In the mediobasal hypothalamus (MBH) of DW-HFD rats, but not SW-HFD animals, leptin increased the ratios of pSTAT3/STAT3, pPI3K/PI3K, pAKT/AKT and pERK/ERK (Fig. 1k), indicating the activation of leptin receptor signalling. Altogether, these results indicate that prolonged suckling enhances the responsiveness to leptin of HFD-fed rats.
Prolonged suckling activates brown adipose tissue thermogenesis
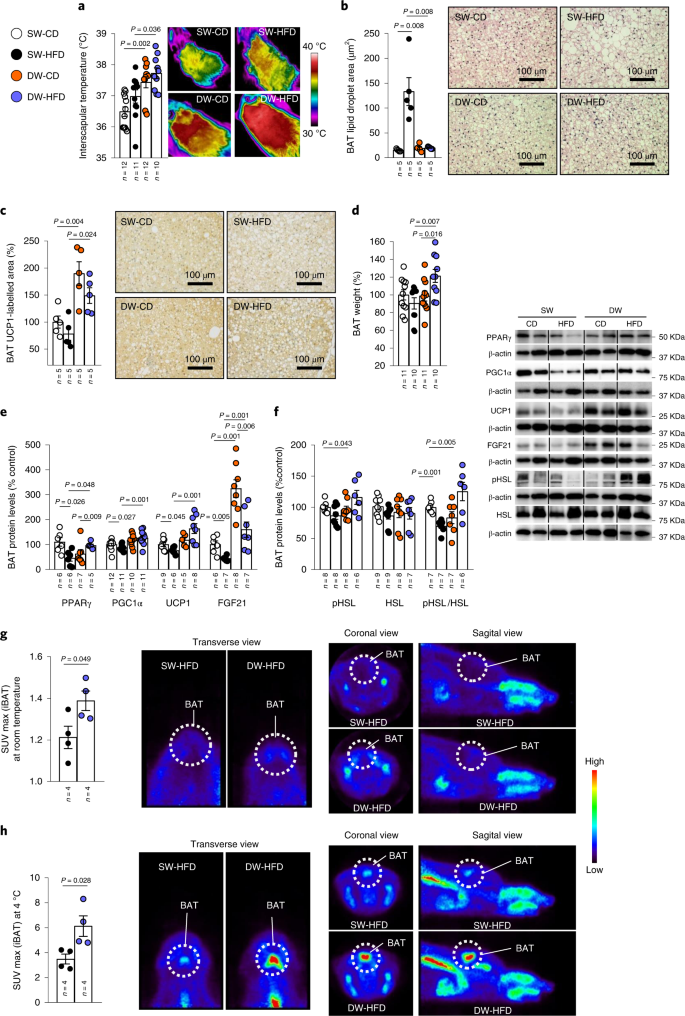
In keeping with the lower body weight and increased energy expenditure seen in DW-HFD rats, these animals also displayed increased interscapular temperature and weight of BAT with respect to the SW-HFD group (Fig. 3a). Furthermore, histological analysis revealed smaller lipid droplets in the BAT of this group, comparable to those in CD-fed animals (Fig. 3b). In agreement with this, BAT weight and immunolabelling for uncoupling protein 1 (UCP1), the main BAT thermogenesis marker, were higher in DW rats fed with either a CD or HFD than in SW rats (Fig. 3c,d). Consistent with the histological data, protein levels of UCP1 were elevated in DW-HFD compared to SW-HFD rats, and the same pattern was observed for other proteins such as peroxisome proliferator-activated receptor-γ coactivator-1-α (PGC1α), peroxisome proliferator-activated receptor-γ (PPARγ) and FGF21, which are predictive of thermogenesis23 (Fig. 3e). In addition, protein levels of phosphorylated hormone-sensitive lipase (pHSL), a lipolytic marker, and the ratio of pHSL/HSL was also increased in the BAT of DW-HFD rats (Fig. 3f).
a–h, Effects of delayed weaning shown on infrared thermal images and quantification of BAT interscapular (iBAT) temperature (n = 10–12; a); quantification of lipid droplet cross-sectional area in BAT (n = 5; b); quantification of immunolabelling for UCP1 in BAT (n = 5; c); BAT weight (n = 10–11; d); BAT protein levels of PPARγ, PGC1α, UCP1 and FGF21 (n = 5–12; e) and pHSL, HSL and pHSL/HSL (n = 6–9; f); 18F-FDG uptake analysis (standardized uptake value (SUV max)) of rats at room temperature (n = 4; g); and 18F-FDG uptake analysis of rats at 4 °C (n = 4; h). Protein data were expressed as percentages in relation to control (SW-CD) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group indicated in each figure. Exact P values are shown. Statistical differences were determined by one-way ANOVA (normal data and homogeneity of variances) followed by Tukey’s post hoc multiple-comparison test (a and c) or a two-sided Student’s t-test (normal data; g and h) or a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; b and d–f).
Source data
In agreement with the increased BAT thermogenesis, DW-HFD animals showed increased glucose uptake in the BAT at room temperature (Fig. 3g) and after cold exposure (Fig. 3h), as indicated by 18F-fluoro-2-deoxy-d-glucose (18F-FDG) uptake in BAT. The data from positron emission tomography-computed tomography (PET/CT) confirmed the activation of the thermogenic programme elicited by prolonged breastfeeding in animals fed an HFD.
Given the activation of the thermogenic programme in the BAT of DW-HFD rats, we then assessed the potential thermogenic role of browning in WAT. The lipid content of the subcutaneous adipose tissue (SAT), as measured by the area covered by lipid droplets, was decreased in DW-HFD compared to SW-HFD rats (Extended Data Fig. 3a). Accordingly, immunolabelling for UCP1 and protein levels of both UCP1 and PGC1α were increased in DW-HFD rats (Extended Data Fig. 3b,c), indicating an increase in browning of WAT. In addition, the pHSL/HSL ratio, a surrogate marker of lipolysis, was also upregulated in DW-HFD rats (Extended Data Fig. 3d).
Interestingly, hepatic steatosis, a hallmark commonly observed in DIO animals, was also ameliorated in DW-HFD rats (Extended Data Fig. 4a). We therefore measured levels of FGF21, a hepatokine with a potent capacity to stimulate thermogenic activity23 and reduce hepatic steatosis24. Hepatic and plasma levels of FGF21 were significantly increased in DW rats (Extended Data Fig. 4b,c), suggesting that FGF21, which is predominantly produced by the liver, might play a role in reversing or preventing HFD-induced changes in animals suckled for prolonged periods.
To avoid the possible effect of the extra week of HFD feeding in the SW-HFD model on thermogenesis, an additional short experiment was performed. After the weaning, SW-HFD rats were fed a CD for 1 week, and then were exposed to an HFD for another week (Extended Data Fig. 5a). So, in this model, both groups (SW and DW) were exposed to 1 week of HFD feeding, and the only difference among both groups was the additional exposure to breastfeeding for 1 week in the DW group (Extended Data Fig. 5a). DW-HFD rats presented lower body weight, higher interscapular temperature and increased expression of the thermogenic markers UCP1 and PGC1a in BAT (Extended Data Fig. 5b–n). It is also important to note that at 4 weeks of age, after the introduction of HFD, although the body weight of both DW and SW was similar, the interscapular temperature was already increased, indicating that the effects of breastfeeding on BAT thermogenesis precede differences in body weight.
Hepatic FGF21 knockdown prevents prolonged suckling effects
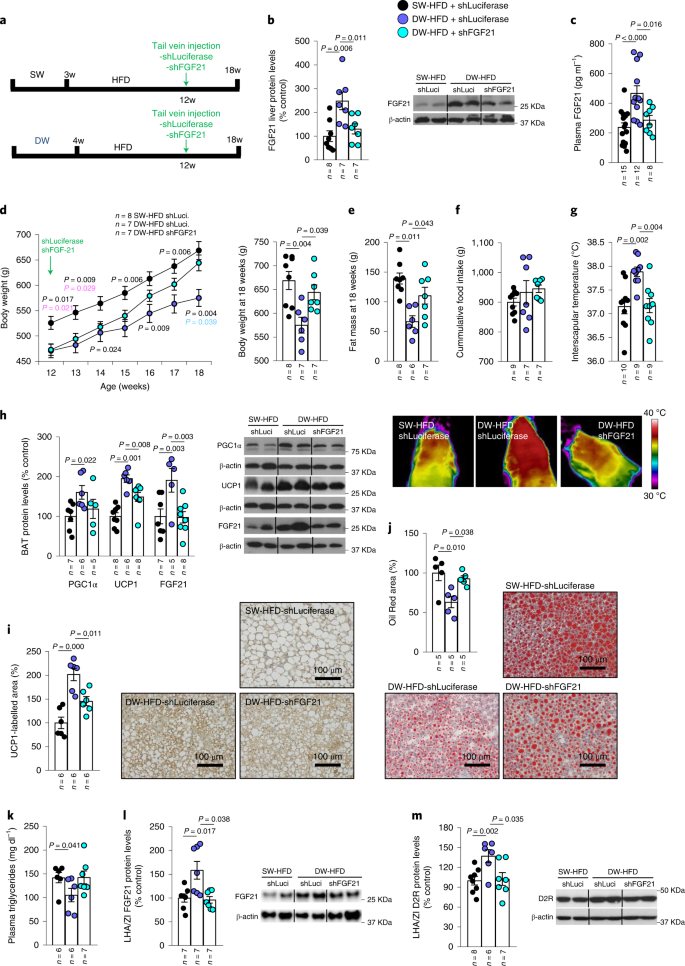
To determine whether the increased hepatic FGF21 production demonstrated above was causally linked to the effects of prolonged suckling on DIO, Fgf21 was reduced by injecting a lentiviral vector encoding an shRNA against Fgf21 into the tail vein of DW-HFD rats (Fig. 4a), a technique previously shown to be effective in selectively targeting the liver25. Lentiviral shRNA delivery normalized the increased hepatic expression and circulating levels of FGF21 observed in DW-HFD rats to SW-HFD levels (Fig. 4b,c). While DW-HFD rats gained less weight than their controls, as seen above (Fig. 1b), the hepatic knockdown of FGF21 partially blunted this effect (Fig. 4d) as well as the decrease in adiposity (Fig. 4e), without affecting feeding behaviour (Fig. 4f). In addition, the increased interscapular temperature displayed by DW-HFD rats was also reversed when hepatic FGF21 was reduced (Fig. 4g). In accordance with the decreased interscapular temperature, the reduction in FGF21 levels also reversed the increased expression of the BAT thermogenesis markers PGC1α and UCP1 induced by prolonged suckling (Fig. 4h) as well as UCP1 immunolabelling in BAT (Fig. 4i). In addition, the reduction of hepatic Fgf21 reversed the increased FGF21 levels in BAT (Fig. 4h). The knockdown of Fgf21 in DW-HFD rats also reversed the reduction of lipid accumulation in the liver, as shown by Oil Red O staining (Fig. 4j) and lowered circulating triglycerides (Fig. 4k). Together, these results indicate that the beneficial effects of prolonged suckling are mediated by an increase in liver FGF21 production.
a, Timeline of the experimental protocol. b–m, Effect of infection with adenoviral particles encoding shFgf21 in the tail vein of 12-week-old rats fed an HFD after prolonged suckling on liver protein levels of FGF21 (b); plasma FGF21 levels (c); body weight (d); fat mass (e); cumulative food intake (f); infrared thermal images and quantification of BAT interscapular temperature (g); BAT protein levels of PGC1α, UCP1 and FGF21 (h); quantification of immunolabelling for UCP1 in BAT (i); Oil Red area in the liver (j); plasma triglycerides (k); LHA/ZI protein levels of FGF21 (l); and LHA/ZI protein levels of D2R (m). Protein data were expressed as percentages in relation to control (SW-HFD shLuciferase) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by one-way ANOVA (normal data and homogeneity of variances) followed by Tukey’s post hoc multiple-comparison test (c–e, g and i–k) or a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; b, f, h, l and m).
Source data
The next step was to elucidate why prolonged suckling increased plasma concentrations of FGF21. To test if the increased FGF21 levels in the offspring were a consequence of the transfer of maternal FGF21 through the milk, FGF21 levels were analysed in plasma and milk from the two different groups of mothers—those nursing the animals for the standard period and mothers of animals subjected to delayed weaning. The body weight of the two groups of mothers was the same at weaning (Extended Data Fig. 6a), and FGF21 levels in plasma or milk were also similar (Extended Data Fig. 6b,c). These findings indicated that the different levels observed in FGF21 were not due to an increased transference of FGF21 from prolonged suckling mothers to the pups.
We measured protein levels of FGF21 in the liver (Extended Data Fig. 6d) and BAT (Extended Data Fig. 6e) of pups and found that they were augmented just after weaning in those animals after extended suckling compared to those suckling for a normal period. Thus, the results indicated that the additional week of breastfeeding was increasing the production of FGF21 levels in the pups. Because fatty acids were proposed as the main regulators of FGF21 expression23, we measured circulating non-esterified fatty acids and found that their levels were higher in those animals under prolonged breastfeeding (Extended Data Fig. 6f).
Brain dopaminergic D2 receptor mediates the effects of prolonged suckling
Energy metabolism and feeding behaviour are regulated by hypothalamic neurons, which respond to circulating metabolic signals26,27,28,29.
Interestingly, we have recently described a hypothalamic mechanism regulating BAT thermogenesis that involves the expression of the dopaminergic D2 receptor (D2R) specifically in the lateral hypothalamic area (LHA) and zona incerta (ZI) but no other hypothalamic regions30. To test whether this dopaminergic mechanism could be involved in the FGF21-dependent metabolic effects of prolonged suckling, we measured the protein levels of FGF21 and D2R in the LHA/ZI.
Indeed, DW-HFD rats showed increased levels of FGF21 in the LHA/ZI compared with SW-HFD animals, which were blunted when hepatic Fgf21 was knocked down (Fig. 4l). Intriguingly, D2R levels, but not orexin, were also increased in the LHA/ZI of DW-HFD compared with SW-HFD animals (Extended Data Fig. 7a) but downregulated again when hepatic Fgf21 production was blocked (Fig. 4m). Overall, these results support the view that FGF21 overproduction during extended suckling reaches hypothalamic dopaminergic circuits to mediate increased BAT thermogenesis.
Reconfirming the relevance of hepatic Fgf21 on D2R expression in the LHA/ZI, mice lacking Fgf21 in the liver, which showed lower interscapular temperature and lower levels of UCP1 in BAT when exposed to cold (Extended Data Fig. 7b,c), also displayed lower protein levels of D2R in the LHA/ZI (Extended Data Fig. 7d).
FGF21 administration mimics prolonged suckling effects
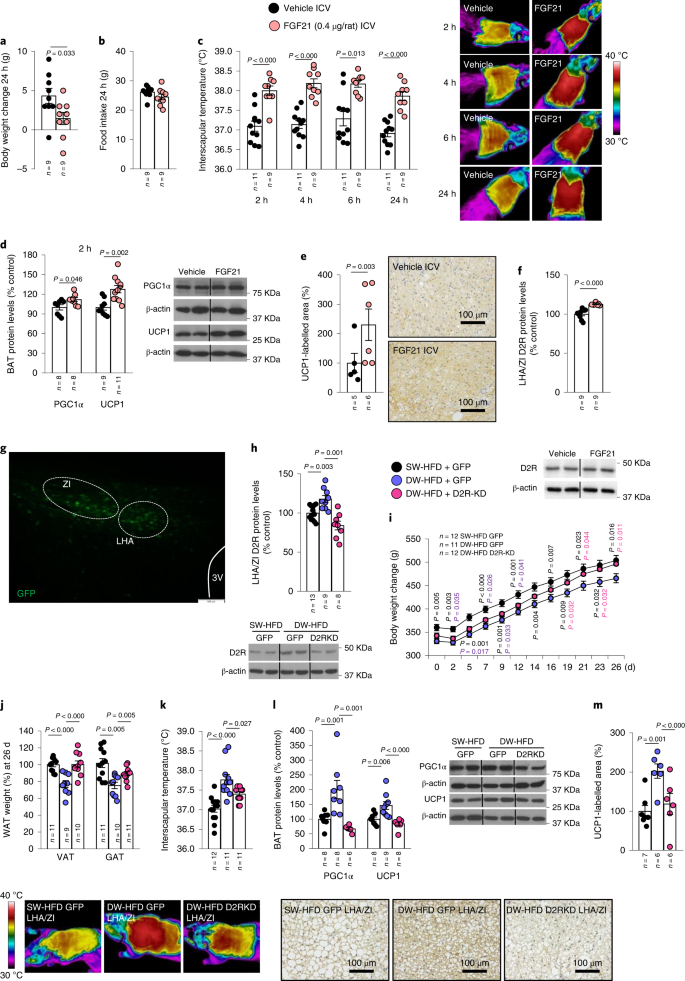
To further elucidate the effects of FGF21, we then tested the effects of centrally administered FGF21 on metabolic parameters and D2R expression in the LHA/ZI in lean control rats subjected to normal weaning and diet. ICV administration of FGF21 at a dose of 0.4 µg per rat reduced body weight after 24 h (Fig. 5a), an effect that was independent of food intake (Fig. 5b). FGF21 administration also induced a sustained increase in interscapular temperature after 2 h of its administration until at least 24 h, indicating increased BAT thermogenesis (Fig. 5c). Accordingly, PGC1α and UCP1 levels as well as UCP1 immunolabelling were increased in the BAT of rats that were administered ICV FGF21 (Fig. 5d,e). In addition, ICV FGF21 led to increased D2R expression in the LHA/ZI as expected (Fig. 5f).
a–f, Effect of ICV injection of FGF21 (0.4 µg per rat) in male rats fed a CD on body weight change at 24 h (a); food intake at 24 h (b); infrared thermal images and quantification of BAT interscapular temperature at 2, 4, 6 and 24 h (c); BAT protein levels of PGC1α and UCP1 (d); quantification of immunolabelling for UCP1 in BAT (e) and LHA/ZI protein levels of D2R (f). g, Representative photomicrograph of a brain section showing GFP expression following injection of viral vectors that encode GFP expression precisely into the LHA/ZI; scale bar, 0.1 mm. h–m, Effect of injecting adenoviral particles encoding GFP or shD2R (leading to D2R knockdown; KD) into the LHA/ZI of rats fed an HFD following prolonged suckling on LHA/ZI protein levels of D2R (h); body weight change (i); WAT weight in terms of weight of VAT and GAT (j); infrared thermal images and quantification of BAT interscapular temperature (k); BAT protein levels of PGC1α and UCP1 (l); and quantification of immunolabelling for UCP1 in BAT (m). Protein data were expressed as percentages in relation to control (vehicle) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by one-way ANOVA (normal data and homogeneity of variances) followed by Tukey’s post hoc multiple-comparison test (i and k) or to a two-sided Student’s t-test (normal data; a–c), a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; d–f, h, j, l and m).
Source data
D2 receptor in LHA/ZI mediates prolonged suckling effects
Given our observation that D2R in the LHA/ZI is increased in DW-HFD animals (Fig. 4m) and induced by FGF21 administration (Fig. 5f), we next sought to determine whether this increase in LHA/ZI D2R levels contributed to the effect of extended suckling on HFD-induced weight gain. For this, D2r expression was reduced by injecting an adeno-associated virus (AAV) encoding an shRNA against D2r (shD2r) or a scrambled sequence in addition to GFP into the LHA/ZI of DW-HFD rats30 (Fig. 5g). shD2r treatment reduced D2R protein expression in the LHA/ZI of DW-HFD rats to levels similar to those in SW-HFD animals (Fig. 5h). It also prevented the reduction in weight gain and adiposity induced by DW in HFD rats (Fig. 5i,j), without affecting feeding behaviour (Extended Data Fig. 8a). Consistent with these changes, the stimulatory effects of prolonged suckling on interscapular temperature, protein levels of UCP1 and PGC1α and UCP1 immunolabelling in BAT were all partially or wholly blunted by D2r silencing in the LHA/ZI (Fig. 5k–m). In addition, the amelioration in liver steatosis and lower plasma triglycerides seen in DW-HFD rats was also reversed when D2r was reduced (Extended Data Fig. 8b,c). These results indicate that D2R in the LHA/ZI mediates the protective role of prolonged suckling against DIO.
Central effects of FGF21 are dependent on D2 receptor in the LHA/ZI
We next investigated whether FGF21-induced D2R expression was required for the biological actions of FGF21. To test this hypothesis, the AAV encoding shD2r was stereotaxically injected into the LHA/ZI of lean control rats that were subsequently administered ICV FGF21. At the titre and time point studied, D2r silencing reversed the effects induced by ICV FGF21 administration on interscapular temperature and protein levels of PGC1α and UCP1 and UCP1 immunolabelling in BAT (Fig. 6a–d). To confirm the requirement of LHA/ZI D2R for the action of FGF21, we then switched to a transgenic mouse model in which D2R-Cre mice were injected into the LHA/ZI with a Cre-dependent AAV encoding either a scrambled RNA (Ad-hSyn-DIO-EGFP) or an shRNA against D2r (Ad-hSyn-DIO-shD2r-EGFP). When FGF21 was subsequently ICV administered in these mice, the FGF21-induced expression of c-Fos in LHA/ZI D2R neurons, indicative of their activation, was strongly decreased in the resulting LHA/ZI neuronal population specifically lacking D2r (Fig. 6e). Importantly, the FGF21-induced decrease in body weight and the increase in interscapular temperature, UCP1 labelling and PGC1α and UCP1 protein levels in BAT were all prevented in mice lacking D2r selectively in the LHA/ZI (Fig. 6f–j and Extended Data Fig. 9a).
a–d, Effect of injecting adenoviral particles encoding GFP or shD2r (D2R-KD) into the LHA/ZI of rats fed a CD and treated with ICV FGF21 on cumulative food intake (a); infrared thermal images and quantification of BAT interscapular temperature (b); BAT protein levels of PGC1α and UCP1 (c); and quantification of immunolabelling for UCP1 in BAT (d). e, Photomicrographs showing the colocalization of GFP and c-Fos in the LHA/ZI of D2r-cre GFP mice treated with ICV FGF21, or shD2R + FGF21 ICV, demonstrating lack of c-Fos activation in LHA/ZI D2r neurons following D2r knockdown. f–j, Effect of injecting an adenoviral vector encoding a scrambled RNA (Ad-hSyn-DIO-EGFP) or an shRNA against D2r (Ad-hSyn-DIO-shD2r-EGFP) in a Cre-dependent manner followed by ICV injection of vehicle or FGF21 into D2r-Cre mice on body weight change (f); cumulative food intake (g); infrared thermal images and quantification of BAT interscapular temperature (h); quantification of immunolabelling for UCP1 in BAT (i); and BAT protein levels of PGC1α and UCP1 (j). Protein data were expressed as percentages in relation to control (GFP vehicle) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided Student’s t-test (normal data; a, d and f–i) or a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; b, c and j).
Source data
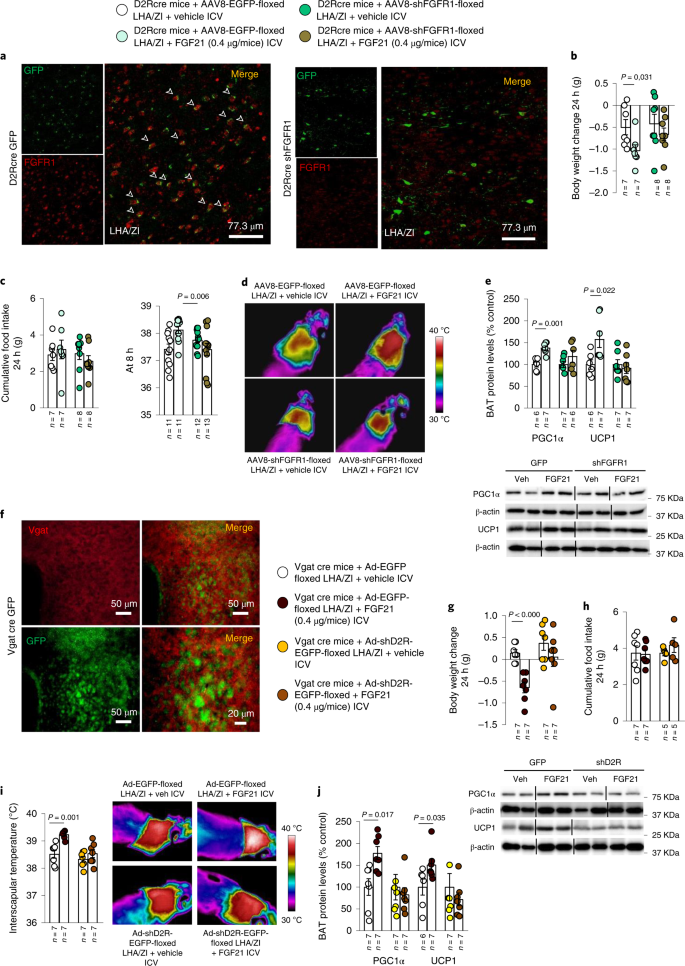
Finally, considering that the actions of FGF21 on body weight and BAT thermogenesis appeared to be dependent on D2r, we assessed whether FGF21 could act directly on LHA/ZI D2r neurons. To this end, the FGF21 receptor (Fgfr1) was selectively reduced in LHA/ZI D2R neurons by stereotaxically delivering AAV8-EGFP-floxed or AAV8-shFgfr1-floxed into the LHA/ZI of D2r-Cre mice (Fig. 7a). Similarly to our previous observations, the FGF21-induced decrease in body weight and increase in interscapular temperature, UCP1 labelling and PGC1α and UCP1 protein levels in BAT were abolished in mice with knockdown of FGFR1 in LHA/ZI D2R neurons (Fig. 7b–e and Extended Data Fig. 9b,c), confirming that FGF21 acts directly on these hypothalamic neurons through its receptor, FGFR1.
a, Photomicrographs showing the colocalization of GFP and FGFR1 in the LHA/ZI of D2r-Cre mice. b–e, Effect of injecting an adenoviral vector encoding a scrambled RNA (AAV-EF1A-EGFP-floxed) or an shRNA against Fgfr1 (AAV8-EGFP-shFgfr1-floxed) in a Cre-dependent manner, followed by ICV injection of vehicle or FGF21 in D2r-Cre mice on body weight change (b); cumulative food intake at 24 h and 8 h (c); infrared thermal images and quantification of BAT interscapular temperature (n = 11–13; d); and BAT protein levels of PGC1α and UCP1 (e). f, Photomicrographs showing the colocalization of GFP and Vgat in the LHA/ZI of D2r-Cre mice. g–j, Effect of injecting an adenoviral vector encoding a scrambled RNA (Ad-hSyn-DIO-EGFP) or an shRNA against D2r (Ad-hSyn-DIO-shD2r-EGFP) in a Cre-dependent manner, followed by ICV injection of vehicle or FGF21 in Vgat-ires-Cre mice on body weight change (g), cumulative food intake (h); infrared thermal images and quantification of BAT interscapular temperature (i); and BAT protein levels of PGC1α and UCP1 (j). Protein data were expressed as percentages in relation to control (GFP vehicle) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided Student’s t-test (normal data; b–d, g and h), or a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; e, i and j).
Source data
D2 receptor in LHA/ZI GABA neurons is required for the FGF21 action
Our previous results have revealed that the LHA/ZI neurons required for the thermogenic action of the hypothalamic dopaminergic system are D2r-expressing GABAergic neurons30. To understand whether the same cell population was also involved in mediating the effects of FGF21, we performed FACS to isolate D2R-expressing GABAergic neurons from the LHA/ZI (Extended Data Fig. 10a). Isolated GABAergic LHA/ZI D2r neurons expressed Fgfr1 mRNA (Extended Data Fig. 10b), suggesting that they could indeed be the effectors of the metabolic actions of FGF21. Accordingly, we knocked down D2r specifically in LHA/ZI GABAergic neurons by delivering Ad-EGFP-floxed (controls) or Ad-shD2r-EGFP-floxed into the LHA/ZI of Vgat-Cre mice, as demonstrated by the co-labelling of GFP and Vgat (Fig. 7f). While ICV administration of FGF21 in control mice induced the expected decrease in body weight and an increase in interscapular temperature, UCP1 labelling and PGC1α and UCP1 protein levels in BAT, without altering food intake, these changes were prevented in mice in which D2R was selectively reduced in LHA/ZI GABA neurons (Fig. 7g–j and Extended Data Fig. 10c,d). These findings demonstrate that the metabolic effects of FGF21 are mediated by D2r-expressing GABAergic neurons of the LHA/ZI.
FGF21 is shuttled into the hypothalamus by tanycytes
Finally, we became curious as to the putative route by which peripheral FGF21 could gain access to its target neurons in the hypothalamus. The main gateway for circulating metabolic signals into the hypothalamus is thought to be the median eminence, a circumventricular organ lining the floor of the third ventricle and characterized by capillaries with a fenestrated endothelium. While metabolic signals such as leptin and ghrelin enter the median eminence parenchyma by passive diffusion through the fenestrated capillaries31,32 to reach deeper hypothalamic structures, these signals require active transport by specialized hypothalamic glia named tanycytes32,33.
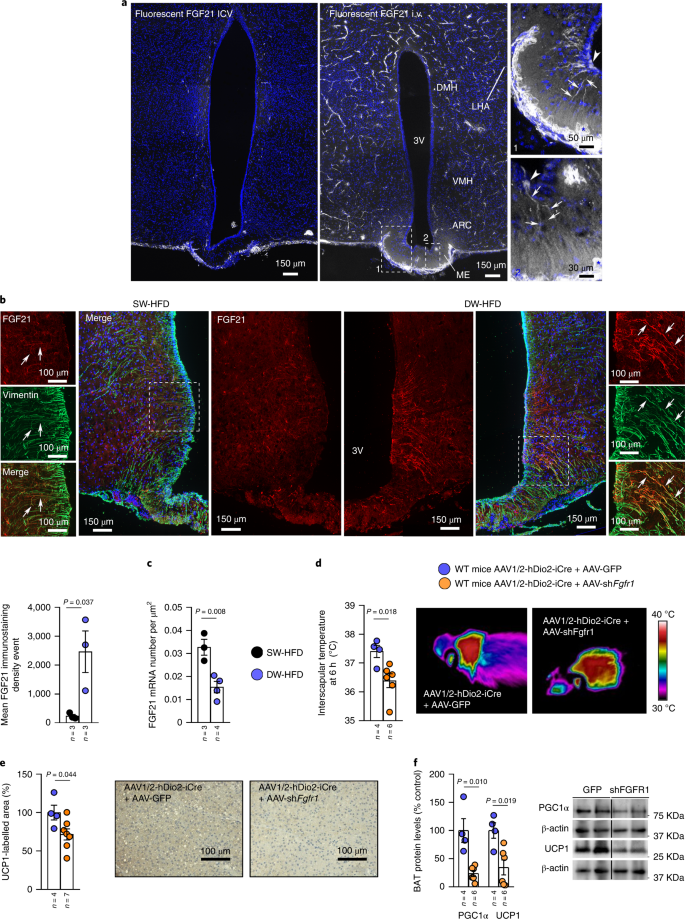
Accordingly, when fluorescently labelled FGF21 was injected through the jugular vein, but not by the ICV route, of control wild-type mice, it was detected in tanycytes of the median eminence (Fig. 8a), suggesting that the same tanycytic shuttle is used to transport hepatic FGF21 into the hypothalamus as is used by other metabolic signals. Interestingly, FGF21 immunoreactivity in DW-HFD rats was significantly increased in tanycytes when compared to SW-HFD rats (Fig. 8b). This increase in FGF21 immunoreactivity in tanycytic processes expressing vimentin, a marker of tanycytes is unlikely due to an upregulation of FGF21 synthesis by the tanycytes themselves because tanycytic Fgf21 transcripts were seen to be markedly downregulated in DW-HFD rats (Fig. 8c).
a, Representative photomicrographs of the tuberal region of the hypothalamus of a wild-type mouse showing tanycytic processes (arrows) and cell bodies (arrowheads) labelled by fluorescent FGF21 in the median eminence (inset), 1 min after injection into the jugular vein or after the ICV injection. b,c, Immunoreactivity of FGF21 is increased in tanycytes of SW-HFD and DW-HFD rats (n = 3; b); mRNA expression of FGF21 in SW-HFD and DW-HFD rats (n = 3; c). d–f, Effect of injecting an AAV1/2 into the lateral ventricle expressing Cre recombinase under transcriptional control of the hDio2 promoter (AAV1/2-hDio2-iCre) together with an AAV-GFP-floxed or an shRNA against Fgfr1 (AAV-GFP-shFgfr1-floxed) in wild-type (WT) mice on infrared thermal images and quantification of BAT interscapular temperature (n = 4–6; d); quantification of immunolabelling for UCP1 in BAT (n = 4–6; e); BAT protein levels of PGC1α and UCP1 (n = 4–6; f). Protein data were expressed as percentages in relation to control (GFP) animals. β-actin was used to normalize protein levels. Dividing lines indicate splicing within the same gel. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided Student’s t-test (normal data; b–e), or a two-sided Mann–Whitney U test (non-normal data and non-homogeneous variance; f).
Source data
Next, to test whether FGFR1-mediated transport of FGF21 through tanycytes may be required for the central regulation of thermogenesis, we inhibited Fgfr1 expression specifically in tanycytes using AAVs. We gave an ICV injection to wild-type mice with AAV1/2 into the lateral ventricle expressing Cre recombinase under transcriptional control of the human type 2 iodothyronine deiodinase (hDio2) promoter (AAV1/2-hDio2-iCre) together with Cre-dependent AAVs expressing GFP or an shRNA against Fgfr1. The efficiency of the approach was demonstrated by assessing the colocalization between GFP and vimentin (Extended Data Fig. 10e). One month after AAV injection, mice were exposed to cold for 6 h and BAT was collected. Mice expressing the Fgfr1 shRNA in tanycytes showed a lower temperature in the BAT after cold exposure (Fig. 8d), which was consistent with the lower levels of the thermogenic markers PGC1α and UCP1 in BAT (Fig. 8e,f). These results raise the intriguing possibility that FGFR1-mediated FGF21 tanycytic shuttles may be required for the central action of liver FGF21 on BAT activity.