According to a new study published in Lancet infectious diseaseThe vaccine for Eastern Equine Encephalitis Virus (EEEV), Western Equine Encephalitis Virus (WEEV) and Venezuelan Equine Encephalitis Virus (VEEV) was found to be safe, well-tolerated among adult volunteers. Published the results of the Phase 1 clinical trial.Also read – Can Stress During Pregnancy Lead To Complications? Talk to the doctor
The vaccine candidate was developed by scientists at the US National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC), which is part of the National Institutes of Health. Also read – What is Salmonella typhimurium – a bacterial infection affecting children in Europe? Is your child at risk? Explain the expert
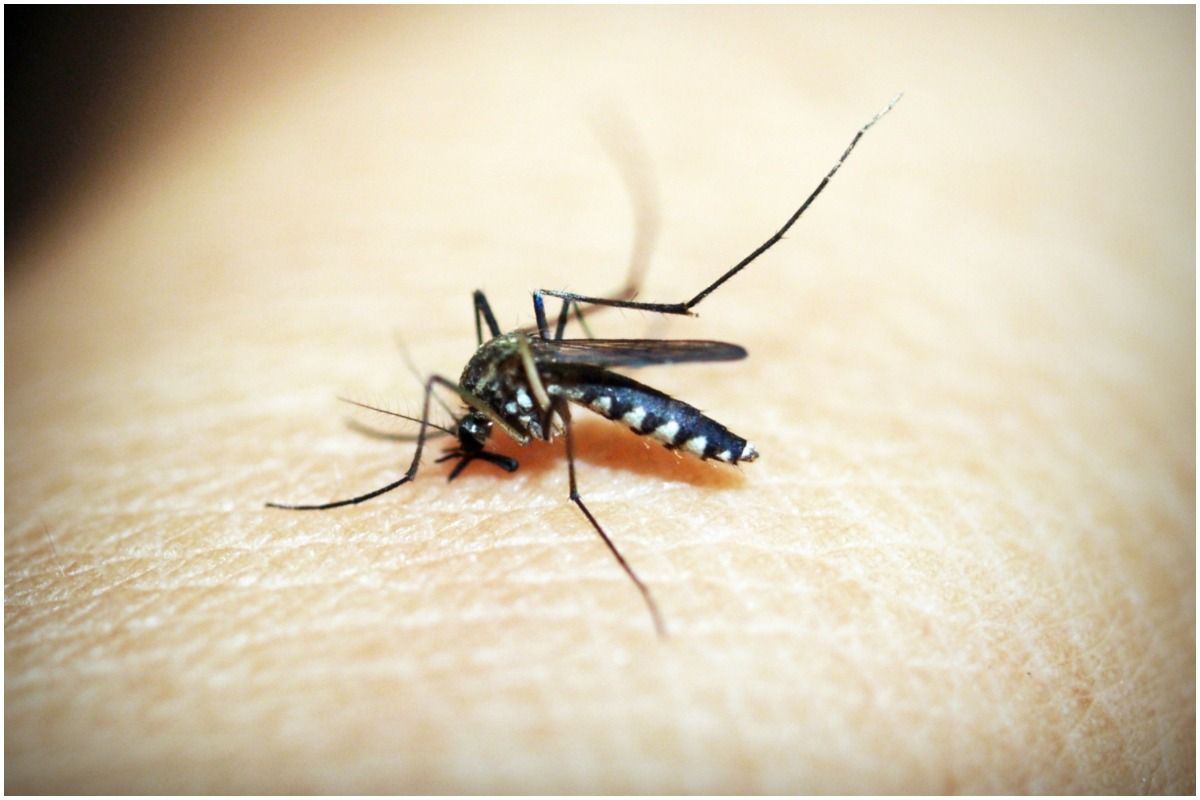
EEEV, WEEV and VEEV are transmitted to humans by the bite of infected mosquitoes. Also read – 5 Reasons Why You Should Include Ladyfinger In Your Diet, Explaining The Health Benefits Of Ladyfinger | Watch the video
Infection with these diseases is rare in humans but can lead to flu-like symptoms and, in some cases, serious neurological damage or death.
However, in certain laboratory conditions, the virus can be transmitted through airborne aerosol drops and cause infections in humans and are therefore classified as predominant pathogens, potential biological agents that pose a threat to national safety and public health.
NIAID Director Anthony Fawcett and his team highlighted the potential usefulness of the EEEV vaccine for people with high occupational risk of disease transmission, including military and laboratory workers.
According to researchers, horses are also susceptible to infection, but horses cannot transmit the virus directly to humans.
The virus has caused repeated, small outbreaks in North, Central and South America, with EEEV outbreaks in the northeastern United States in 2019 resulting in 38 confirmed cases and 15 deaths.
In a study appearing in The Lancet Infectious Diseases, VRC researchers have developed a virus-like pigment (VLP) vaccine candidate (abbreviated WEVEE) that uses proteins from the outer shell of EEE, WEE and VEE viruses to respond to the immune response. VLPs do not contain the genetic material needed to replicate the virus inside cells, so VLPs cannot cause infection.
For the Phase 1 clinical trial, 30 healthy adult volunteers between the ages of 18 and 50 received different doses (6, 30, or 60 micrograms) of the WEVEE vaccine by intramuscular injection.
Participants then returned after eight weeks to receive the same dose as Boost. Some participants also achieved the formulation of an experimental vaccine with the addition of an alum supplement to enhance the immune response.
The team reports that the vaccine is safe, well tolerated and promotes lasting resistance to all three viruses.
Participants receiving a 30-microgram dose with adjuvant had the highest neutral antibody response. The authors note that the findings support further clinical evaluation of the vaccine candidate.
NIAID has granted commercial license to life science company Emergent Biosolutions in Maryland, USA for advanced development of WEVEE vaccine candidate.
(IANS)