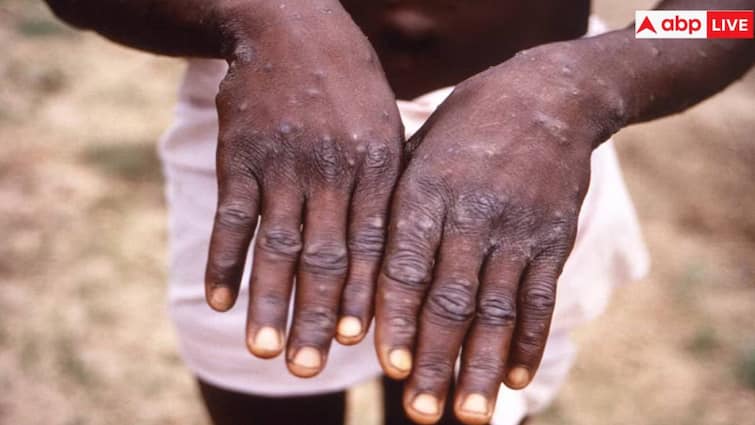
The World Health Organization on Friday approved Bavarian Nordic’s MVA-BN vaccine as the first shot against mumps. The World Health Organization announced on Friday that it had granted its first authorization for the use of a mumps vaccine in adults. This was described as an important step in the fight against the disease in Africa and beyond. The prequalification of the vaccine by Bavarian Nordic A/S means that donors such as GAVI Vaccine Alliance and UNICEF can buy it. But supply is limited because there is only one manufacturer.
Bavarian Nordic Vaccines (BAVA.CO)
The World Health Organization announced Friday that it has approved the Bavarian Nordic vaccine (BAVA.CO) as the first shot against MPOX. The approval, called prequalification, is an official list of medicines used as a reference for developing countries to purchase. Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, said: “This first prequalification of a vaccine against MPOX is an important milestone in our fight against the disease, both in the context of the current outbreaks in Africa and in the future.” p>
The vaccine will be administered in these countries, including in Africa
WHO Director-General Tedros Adhanom Ghebreyesus said: “This first prequalification of a Mpox vaccine is an important milestone in our fight against current and future outbreaks in Africa.” The head of the UN health agency called for an immediate increase in the scale of procurement, donations and deployment to get the vaccine to where it is needed, as well as other response measures.
2 doses of vaccine will be administered to people of this age
Under WHO authorization, the vaccine can be given in two doses of two doses to people aged 18 years and older. The approval indicates that although the vaccine is not currently authorized for people under 18 years of age, it can be used in infants, children and adolescents in epidemic areas where the benefits of vaccination outweigh the potential risks.
Disclaimer: Some information provided in the news is based on media reports. Before implementing any suggestion, you must consult the concerned expert.